Compound ID | 1061
Cefsulodin
Synonym(s): CGP 7174/E
Class: Beta-lactam (cephalosporin)
| Spectrum of activity: | Gram-negative |
| Details of activity: | Active against Pseudomonas. Mainly used for research purposes in selective media. |
| Description: | Tosch, W., et al. "In vitro characterization of CGP 7174/E, a Cephalosporin active against Pseudomonas." Current chemotherapy 2 (1978): 843-844. 10th ICAAC p. 843 |
| Institute where first reported: | Takeda Pharmaceuticals |
| Year first mentioned: | 1977 |
| Highest developmental phase: | Phase 4 |
| Development status: | Inactive |
| Reason Dropped: | Poor stability. |
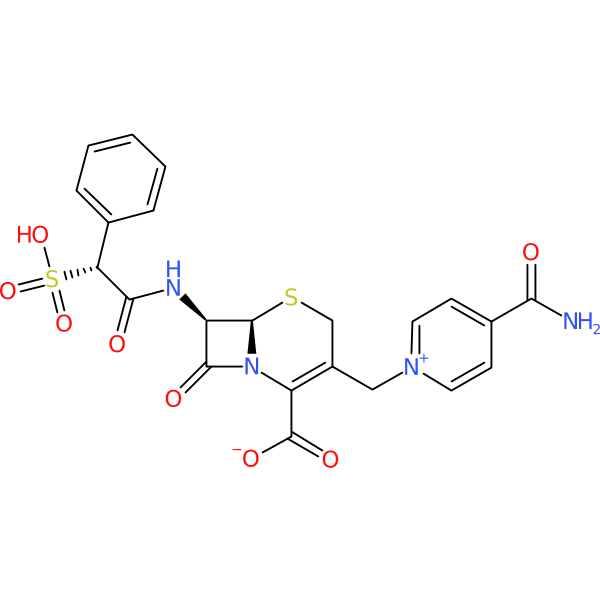
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/656575 |
| Guide to Pharmacology: | cefsulodin |
| Citation: |