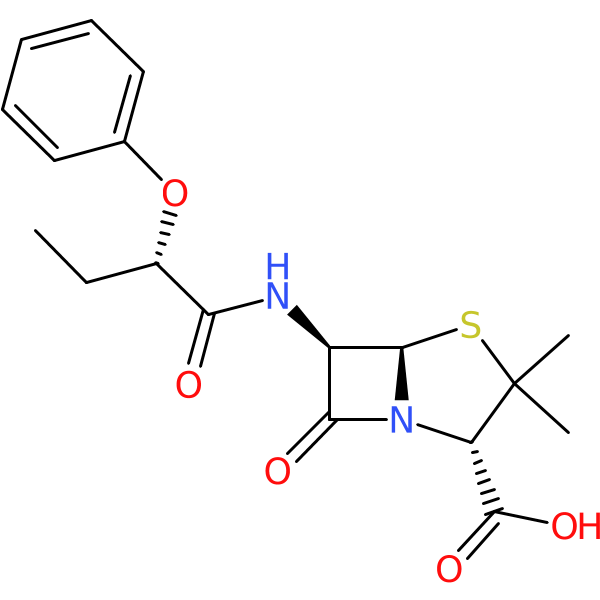
Compound ID | 1145
levopropylcillin
Synonym(s): L-Propicillin | BRL-284
Class: Beta-lactam (penicillin)
| Spectrum of activity: | Gram-positive |
| Details of activity: | Active against Staphylococci but not stable to penicillinase |
| Description: | Godzeski, C, W et al. "An In Vitro Examination of Levopropylcillin" Program and Abstracts of the interscience science Conference on Antimicrobial Agents and Chemotherapy. 1961. |
| Institute where first reported: | Eli Lilly |
| Year first mentioned: | 1961 |
| Highest developmental phase: | Preclinical |
| Development status: | Inactive |
| Reason Dropped: | Resistance and competition from competing drugs |
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/172994 |
| Guide to Pharmacology: | levopropicillin |
| Citation: |