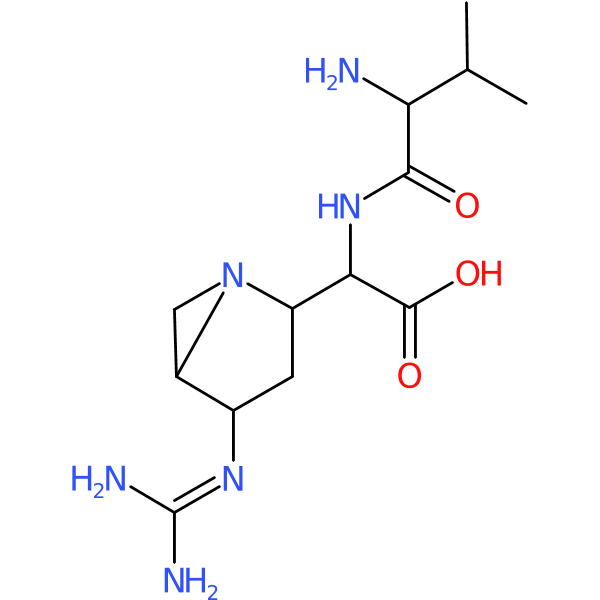
Compound ID | 1184
Ficellomycin
Class: Aziridine
| Spectrum of activity: | Gram-positive & Gram-negative |
| Details of activity: | DNA synthesis inhibitor |
| Institute where first reported: | The UpJohn Company |
| Year first mentioned: | 1976 |
| Highest development stage: | Preclinical |
| Development status: | Active |
| Reason dropped: | High MIC of fermentation broth means it hasn't attracted much attention, but engineered biosynthesis may make it a pheezable antibiotic. |
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/151592 |
| Guide to Pharmacology: | ficellomycin |
| Citations: |
|