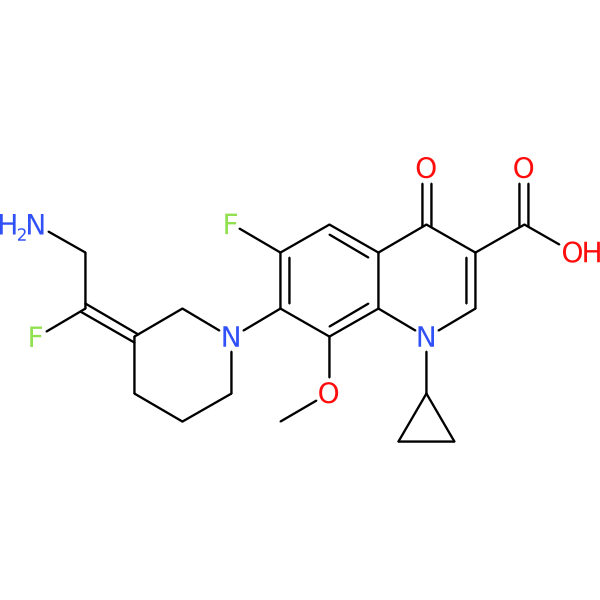
Compound ID | 1210
Avarofloxacin
Synonym(s): JNJ-Q2
Class: Fluoroquinolone
| Spectrum of activity: | Gram-positive |
| Details of activity: | Active against methicillin-resistant Staphylococcus aureus |
| Description: | Broad Spectrum In Vitro Activity of JNJ-Q2, a New Fluoroquinolone. F1-2033. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC. By: Foleno, B |
| Institute where first reported: | Johnson & Johnson, Melinta Therapeutics Inc. |
| Year first mentioned: | 2008 |
| Highest development stage: | Phase 2 (NCT01128530) |
| Development status: | Active |
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/11546234 |
| Guide to Pharmacology: | acorafloxacin |
| Citation: |