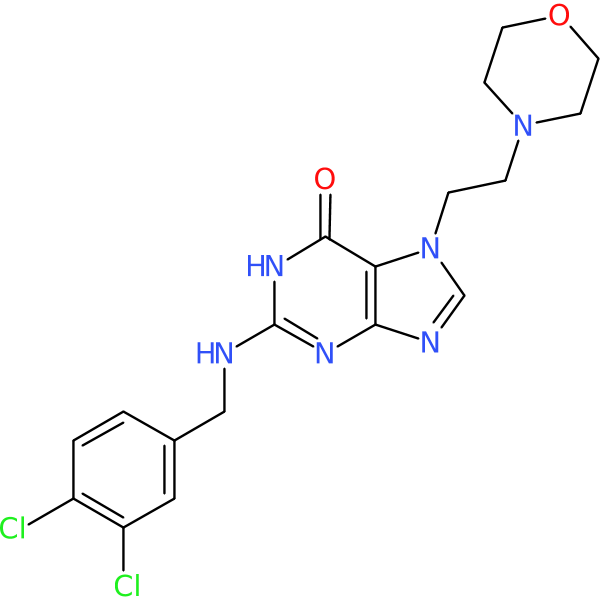
Compound ID | 1231
Ibezapolstat
Synonym(s): ACX-362E
Class: DNA synthesis inhibitor
| Spectrum of activity: | Gram-positive |
| Details of activity: | C. difficile infections |
| Institute where first reported: | Acurx Pharmaceuticals LLC |
| Highest development stage: | Phase 2 |
| Development status: | Active |
| Chemical structure(s): | |||||||||||
|
|