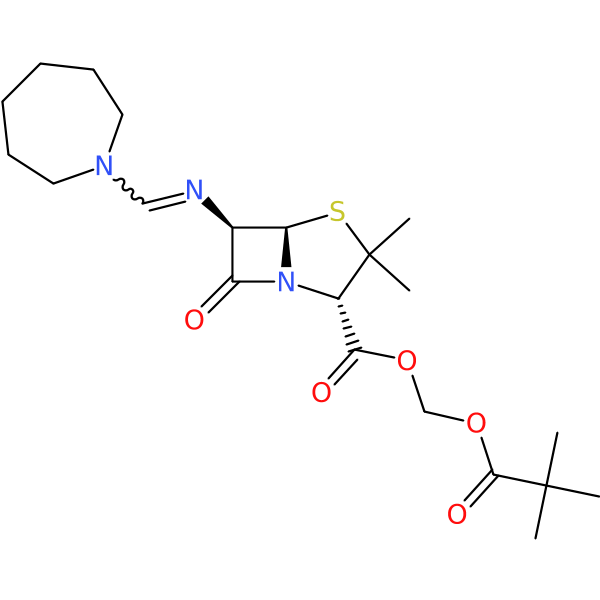
Compound ID | 1340
Pivmecillinam (Prodrug of Amdinocillin)
Synonym(s): Pivya | Amdinocillin pivoxil
Class: Beta-lactam (penicillin)
| Spectrum of activity: | Gram-negative |
| Details of activity: | Used primarily in the treatment of lower UTI's. Also uses the trade names: Selexid, Penomax and Coactabs; binds to bacterial penicillin-binding proteins (PBP) and interferes with bacterial cell wall integrity and synthesis. Active against Enterobacteriaceae incl. ESBL producers |
| Description: | Prodrug of mecillinam. Used for oral therapy of urinary tract infections. |
| Institute where first reported: | Leo Pharmaceutical Products; Utility Therapeutics |
| Year first mentioned: | 1975 |
| Highest developmental phase: | Approved by FDA in 2024 |
| Development status: | Approved |
| Chemical structure(s): | |||||||||||
|
|