Compound ID | 1870
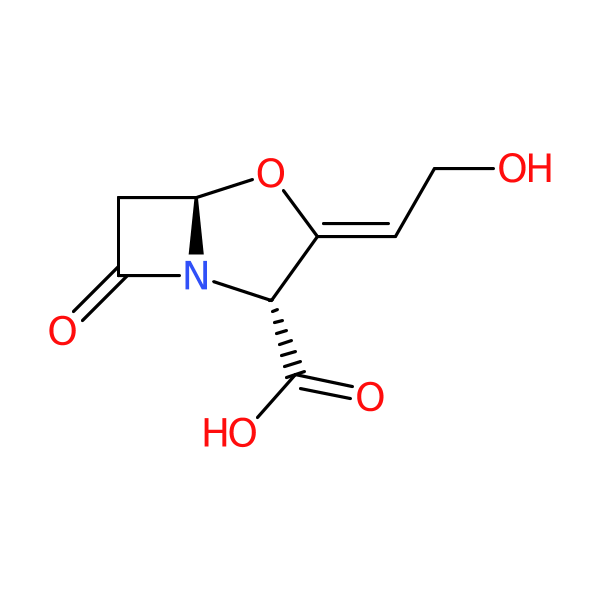
Clavulanic acid
Class: Beta-lactamase inhibitor (clavam, bicyclic beta-lactam without a penicillin or cephalosporin nucleus)
| Details of activity: | Binds irreversibly to beta-lactamases, mainly class A enzymes. No intrinsic antibacterial activity |
| Combined with other compounds: | Yes |
| Description: | Streptomyces natural product. Only available as a combination product, oral and parenteral application |
| Year first mentioned: | 1976 |
| Development status: | Approved, off-patent |
| Chemical structure(s): | |||||||||||
|
|