|
|
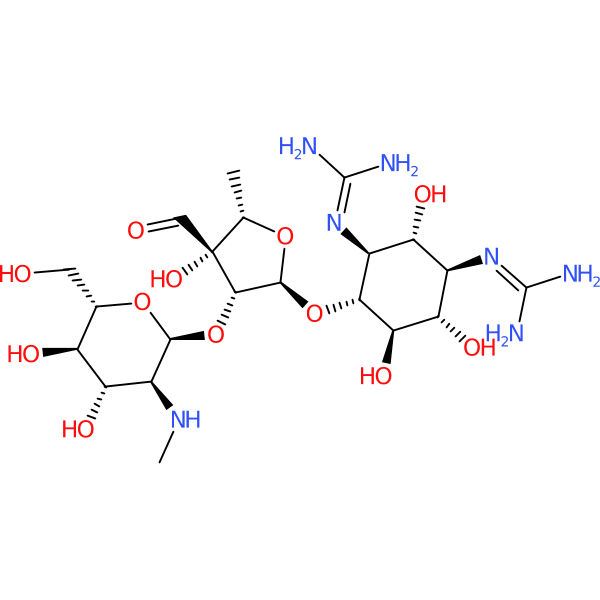
Molecular weight: 581.57
|
|
|
Iso. SMILES:
|
C[C@H]1[C@@]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H]([C@@H]([C@H]2O)O)N=C(N)N)O)N=C(N)N)O[C@H]3[C@H]([C@@H]([C@H]([C@@H](O3)CO)O)O)NC)(C=O)O
|
|
InChI Key:
|
UCSJYZPVAKXKNQ-HZYVHMACSA-N
|
|
Can. SMILES:
|
C[C@H]1[C@](C=O)([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H]([C@@H]([C@H]2O)O)N=C(N)N)O)N=C(N)N)O[C@H]3[C@H]([C@@H]([C@H]([C@H](CO)O3)O)O)NC)O
|
|
InChI:
|
InChI=1S/C21H39N7O12/c1-5-21(36,4-30)16(40-17-9(26-2)13(34)10(31)6(3-29)38-17)18(37-5)39-15-8(28-20(24)25)11(32)7(27-19(22)23)12(33)14(15)35/h4-18,26,29,31-36H,3H2,1-2H3,(H4,22,23,27)(H4,24,25,28)/t5-,6-,7+,8-,9-,10-,11+,12-,13-,14+,15+,16-,17-,18-,21+/m0/s1
|
|


