Compound ID | 2182
TBI-166
Synonym(s): Pyrifazimine
Class: Small molecule antibacterial agent
| Agent Type: | Synthetic; Small molecule; |
| Spectrum of activity: | Antimycobacterial |
| Mechanism of action: | Unknown |
| Target Pathogen: | Active against Mycobacterium sp. |
| Description: | Synthetic compound derived from optimization of riminophenazine analogues |
| Institute where first reported: | Institute of Materia Medica of the Chinese Academy of Medical Sciences |
| Year first mentioned: | 2012 |
| Highest development stage: | Phase 2 (NCT04670120) |
| Development status: | Active (as of 2023) |
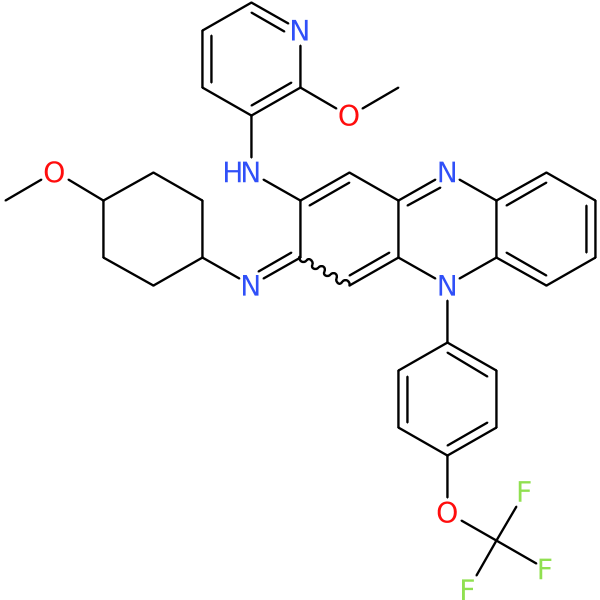
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/53377576 |
| Guide to Pharmacology: | TBI-166 |
| Citations: |
|
| Patent: | WO2012003190A1 |