Compound ID | 2892
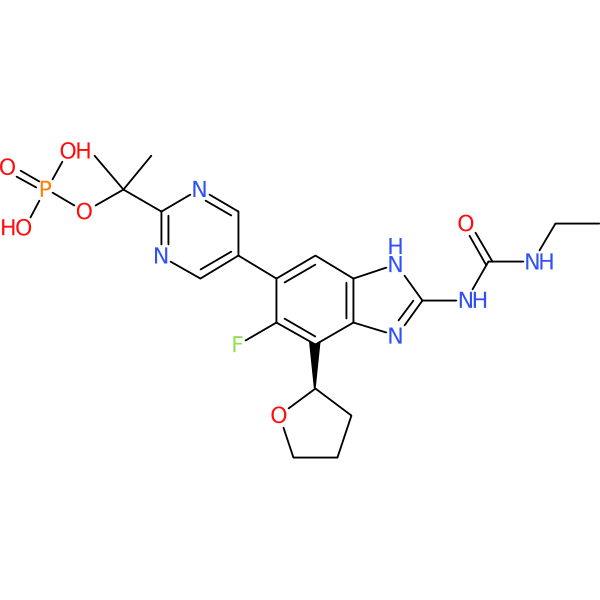
SPR720 (phosphate prodrug of SPR719)
Synonym(s): Fobrepodacin
Class: Small molecule antibacterial agent
| Agent Type: | Synthetic; Small molecule; Direct acting; |
| Spectrum of activity: | Antimycobacterial |
| Mechanism of action: | DNA synthesis inhibitor. DNA gyrase inhibitor (targeting GyrB subunit)/ bacterial topoisomerase inhibitor |
| Target Pathogen: | Active against Mycobacterium avium, Mycobacterium kansasii, Mycobacterium ulcerans, Mycobacterium marinum, and Mycobacterium chimaera |
| Description: | Synthetic compound; for nontuberculous mycobacterial pulmonary infection; rapidly converted to the active moiety SPR719 in vivo |
| Institute where first reported: | Spero Therapeutics, Inc., Cambridge, Massachusetts, USA |
| Year first mentioned: | 2018 |
| Highest development stage: | Phase 2 (NCT05496374) |
| Development status: | Active (as of 2024) |
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/68108988 |
| Guide to Pharmacology: | fobrepodacin |
| Citation: | https://journals.asm.org/doi/10.1128/aac.01208-21 |