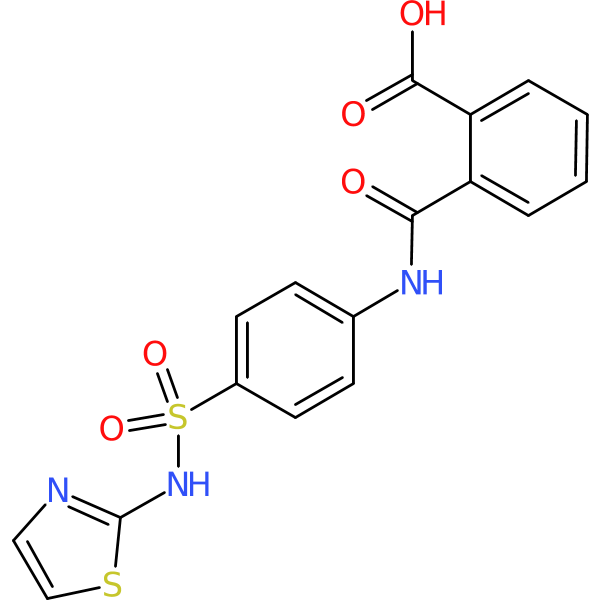
Compound ID | 3271
Phthalylsulfathiazole
Synonym(s): sulfathalidine
Class: Sulfonamide
| Agent Type: | Synthetic; Small molecule; Direct acting; |
| Spectrum of activity: | Gram-positive & Gram-negative |
| Mechanism of action: | Folate synthesis inhibitor. Dihydrofolate reductase inhibitor |
| Target Pathogen: | Active against Escherichia coli, streptococci, and staphylococci |
| Description: | Synthetic compound (sulfa/ sulphonamide drug) |
| Year first mentioned: | 1945 |
| Highest development stage: | Clinical trial in 1945 |
| Development status: | Inactive |
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/4806 |
| Citation: | https://jamanetwork.com/journals/jama/article-abstract/276901 |