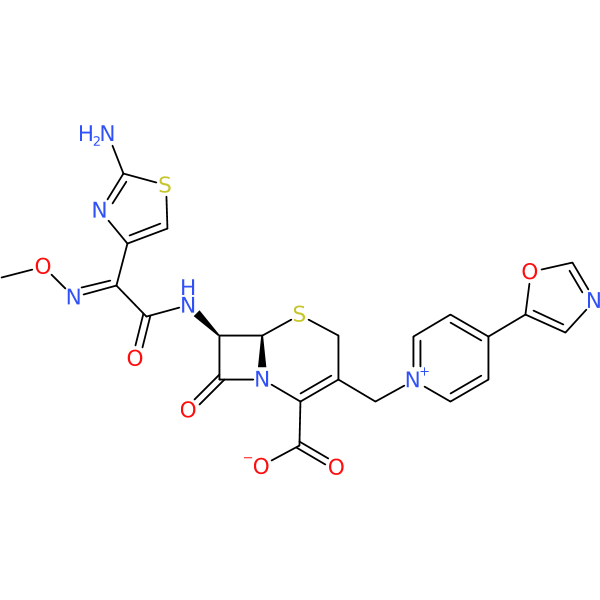
Compound ID | 362
DQ-2556
Class: Beta-lactam (cephalosporin)
| Spectrum of activity: | Gram-positive & Gram-negative |
| Details of activity: | Broad spectrum antibiotic which was comparable or superior to ceftazadime, cefotaxime and cefepime at inhibiting 90% of of Enterobacteriaceae tested. However, MRSA were resistant. Can be used against UTIs |
| Description: | Chin NX, Huang HB and Neu HC. In vitro activity and β-lactamase stability of DQ-2556 compared to other agents. 32nd-Intersci-Conf-Antimicrobial-Agents-Chemother 1992; 176 |
| Institute where first reported: | Daiichi Sankyo Pharmaceutical, Japan |
| Year first mentioned: | 1988 |
| Development status: | Inactive |
| Reason Dropped: | Some frequent side effects: diarrhoea, fever and skin eruptions, was in phase II for parenteral administration in Japan for UTI and then discontinued. This may be due to side effects (2) |
| Chemical structure(s): | |||||||||||
|
|