Compound ID | 3638
Platensimycin
Synonym(s):
Class: Natural product antibiotic
| Agent Type: | Natural product; Small molecule; Direct acting; |
| Spectrum of activity: | Gram-positive |
| Mechanism of action: | Fatty acid synthesis inhibitor. FabF inhibitor |
| Target Pathogen: | Active against methicillin-resistant and -susceptible Staphylococcus aureus, macrolide-resistant Enterococcus faecalis, vancomycin-resistant Enterococcus faecium, and Streptococcus pneumoniae |
| Description: | Natural product from Streptomyces platensis; shows no toxicity towards HeLa cells; shows 4-5 log reduction of Staphylococcus aureus in mouse infection model |
| Institute where first reported: | Merck Research Laboratories, USA |
| Year first mentioned: | 2006 |
| Development status: | Experimental |
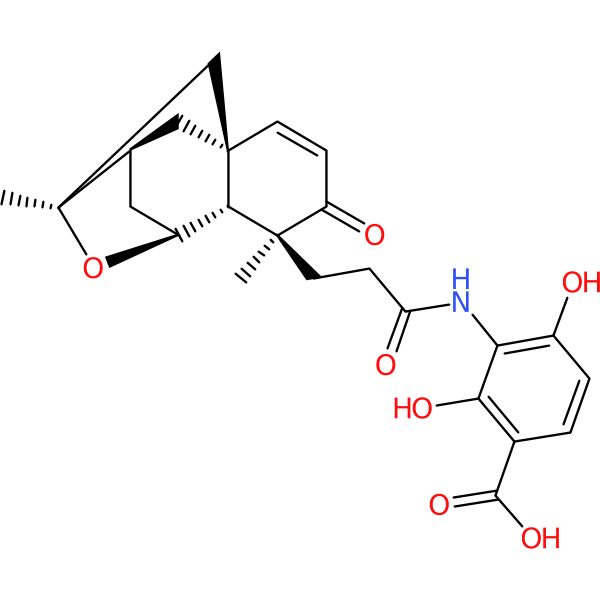
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| Structure link: | https://pubchem.ncbi.nlm.nih.gov/compound/6857724 |
| Citation: | https://www.nature.com/articles/nature04784 |