Compound ID | 3641
Fabimycin
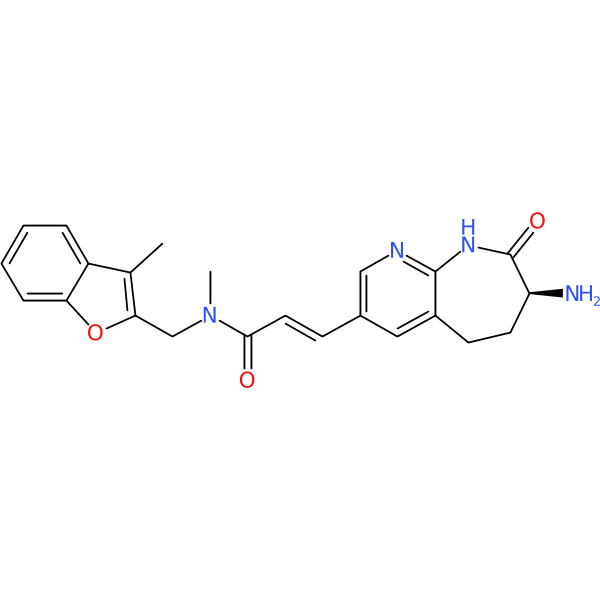
Synonym(s): (2E)-3-[(7S)-7-amino-8-oxo-6,7,8,9-tetrahydro-5H-pyrido[2,3-b]azepin-3-yl]-N-methyl-N-[(3-methyl-1-benzofuran-2-yl)methyl]prop-2-enamide
Class: Small molecule antibacterial agent
| Agent Type: | Synthetic; Small molecule; Direct acting; |
| Spectrum of activity: | Gram-positive & Gram-negative |
| Mechanism of action: | Fatty acid synthesis inhibitor. FabI (Fatty acid biosynthesis: Enoyl-[acyl-carrier-protein] reductase [NADH]) |
| Target Pathogen: | Active against Staphylococcus aureus and moderately active against Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii |
| Propensity to select resistant mutants: | Yes, low at 8x~32x MIC |
| Description: | Synthetic compound based on Debio-1452-NH3 which can enter the Gram-negative outer membrane |
| Institute where first reported: | Department of Chemistry and Carl R. Woese Institute for Genomic Biology, University of Illinois at Urbana−Champaign, Urbana, Illinois 61801, United States |
| Year first mentioned: | 2022 |
| Development status: | Experimental |
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| Citation: | https://pubs.acs.org/doi/10.1021/acscentsci.2c00598 |
| Patent: | WO2022187329 WO2021123372 WO2022268890 |