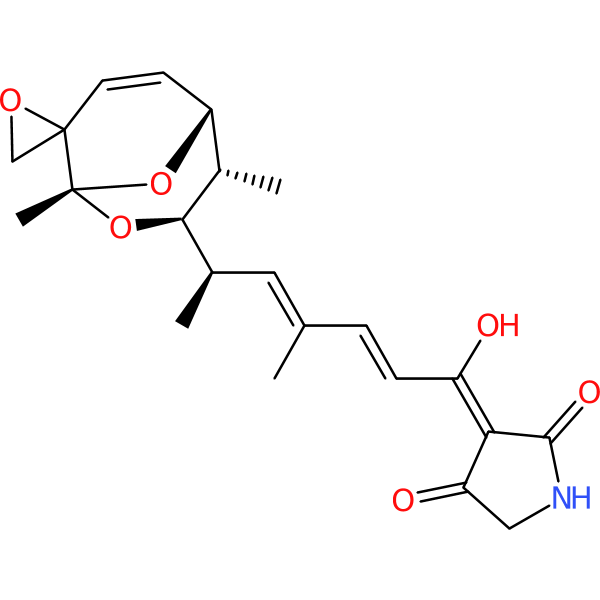
Compound ID | 389
Tirandalydigin
Class: Beta-lactam (penem)
| Spectrum of activity: | Gram-positive & Gram-negative |
| Details of activity: | Bacterial DNA-directed RNA polymerase; structurally similar to both tirandamycin and streptolydigin. Potent against a number of anaerobes. |
| Institute where first reported: | Abbott Laboratories, USA |
| Year first mentioned: | 1987 |
| Highest developmental phase: | Preclinical |
| Development status: | Inactive |
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/54717174 |
| Guide to Pharmacology: | tirandalydigin |
| Citations: |
|