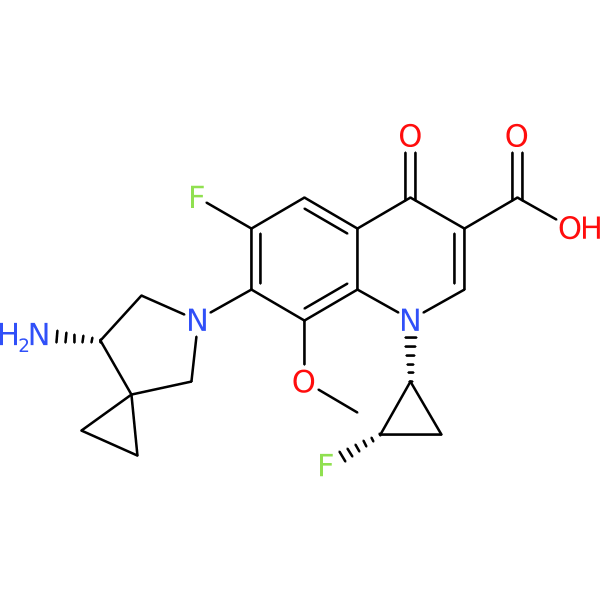
Compound ID | 563
DK-507k
Class: Fluoroquinolone
| Agent Type: | Synthetic; Small molecule; |
| Spectrum of activity: | Gram-positive |
| Mechanism of action: | Unknown |
| Target Pathogen: | Active against methicillin-resistant Staphylococcus aureus and pathogen causing community-acquired pneumoniae |
| Institute where first reported: | Daiichi Pharmaceutical; Pfizer |
| Year first mentioned: | 2001 |
| Highest development stage: | Phase 1 |
| Development status: | Inactive |
| Reason dropped: | Pfizer and Daiichi announced discontinuation of this antibiotic in 2004 after analysis of Phase I trial data |
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/5271826 |
| Guide to Pharmacology: | DK-507k |
| Citation: | https://journals.asm.org/doi/10.1128/aac.47.12.3750-3759.2003 |