Compound ID | 708
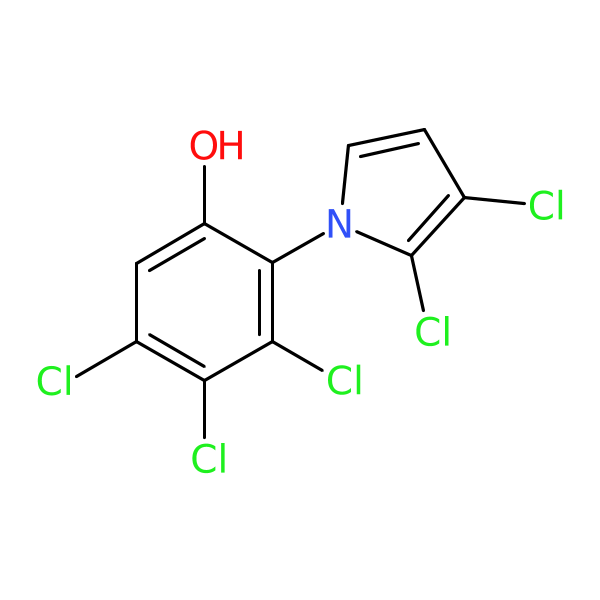
Neopyrrolomycin
Class: Phenylpyrrole
| Spectrum of activity: | Gram-positive & Gram-negative |
| Details of activity: | The base compound was synthesised from 3, 5-dichloroanisole which was then chemically manipulated to generate a number of analogs. Many demonstrated toxicity. The less chlorinated analogue had broad spectrum activity and reduced toxicity. |
| Institute where first reported: | Wasada University, Japan |
| Year first mentioned: | 1994 |
| Highest developmental phase: | Preclinical |
| Development status: | Inactive |
| Chemical structure(s): | |||||||||||
|
|
| External links: | |
| PubChem link: | https://pubchem.ncbi.nlm.nih.gov/compound/125512 |
| Guide to Pharmacology: | neopyrrolomycin |
| Citations: |
|